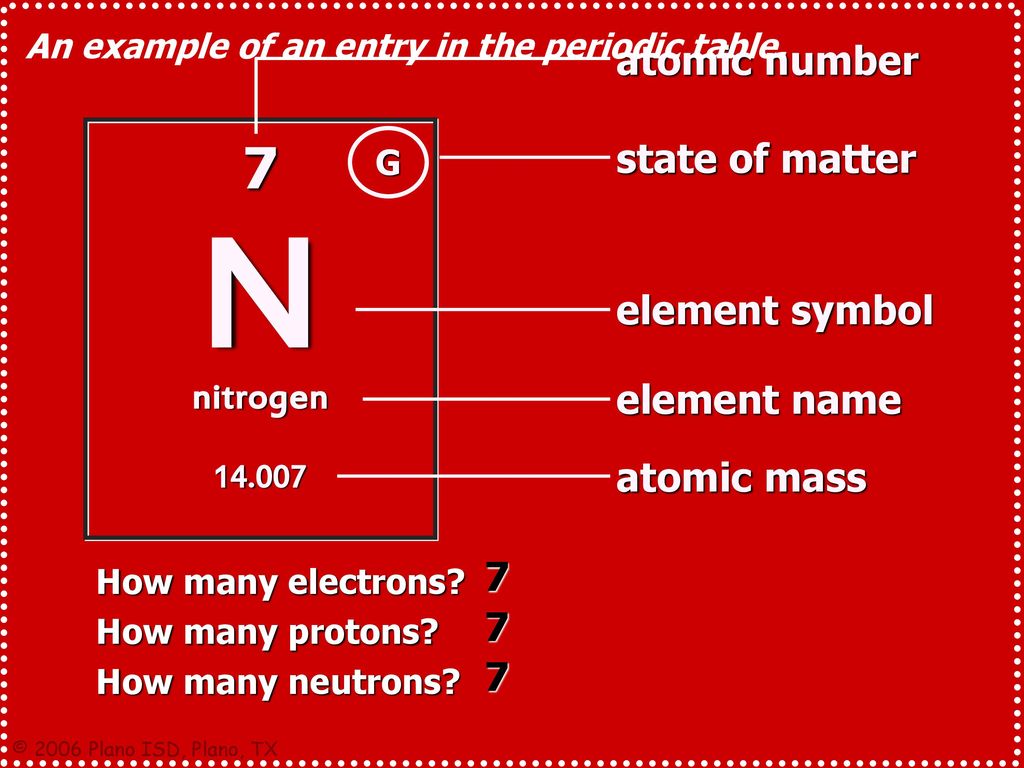
Element Nitrogen - N
- Atomic Mass For Nitrogen
- Atomic Mass And Atomic Number For Nitrogen
- The Atomic Number For Nitrogen
- Symbol For Nitrogen
Comprehensive data on the chemical element Nitrogen is provided on this page; including scores of properties, element names in many languages, most known nuclides of Nitrogen. Common chemical compounds are also provided for many elements. In addition technical terms are linked to their definitions and the menu contains links to related articles that are a great aid in one's studies. Site to download games for mac.
Nitrogen is a chemical element with atomic number 7 which means there are 7 protons in its nucleus. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z. The total electrical charge of the nucleus is therefore +Ze, where e (elementary charge) equals to 1,602 x 10-19 coulombs. Nitrogen is a chemical element which has the symbol N and atomic number 7. Elemental nitrogen is a colourless, odourless, tasteless and mostly inert diatomic gas at standard conditions, constituting 78.1% by volume of Earth's atmosphere. Nitrogen, N, is an odorless, colourless, and tasteless gas. It is a non-metal with one of the. The atomic number is the number of protons in the nucleus of an atom. The number of protons define the identity of an element (i.e., an element with 6 protons is a carbon atom, no matter how many neutrons may be present). The number of protons determines how many electrons surround the nucleus, and it is the arrangement of these electrons that. Answers for atomic number of nitrogen crossword clue. Search for crossword clues found in the Daily Celebrity, NY Times, Daily Mirror, Telegraph and major publications. Find clues for atomic number of nitrogen or most any crossword answer or clues for crossword answers.
Nitrogen has two isotopes, N-14 and N-15, both of which are used in various applications. N-15 is used for the production of the radioisotope O-15 which is used in PET. N-15 is also used to study the uptake of Nitrogen in plants and the metabolism of proteins in the human body. N-14 is used for the production of the PET radioisotope C-11.


Nitrogen Menu
- Nitrogen Page One
- Nitrogen Page Two
- Nitrogen Page Three
Overview of Nitrogen
- Atomic Number: 7
- Group: 15
- Period: 2
- Series: Nonmetals
Nitrogen's Name in Other Languages
- Latin: Nitrogenium
- Czech: Dusík
- Croatian: Dušik
- French: Azote
- German: Stickstoff - r
- Italian: Azoto
- Norwegian: Nitrogen
- Portuguese: Nitrogênio
- Russian: Азот
- Spanish: Nitrógeno
- Swedish: Kväve
Atomic Structure of Nitrogen
- Atomic Radius: 0.75Å
- Atomic Volume: 17.3cm3/mol
- Covalent Radius: 0.75Å
- Cross Section (Thermal Neutron Capture) σa/barns: 1.91
- Crystal Structure: Hexagonal
- Electron Configuration:
- 1s2 2s2p3
- Electrons per Energy Level: 2,5
- Shell Model
- Shell Model
- Ionic Radius: 0.13Å
- Filling Orbital: 2p3
- Number of Electrons (with no charge): 7
- Number of Neutrons (most common/stable nuclide): 7
- Number of Protons: 7
- Oxidation States:±3,5,4,2
- Valence Electrons: 2s2p3
- Electron Dot Model
- Electron Dot Model
Chemical Properties of Nitrogen
- Electrochemical Equivalent: 0.10452g/amp-hr
- Electron Work Function:
- Electronegativity: 3.04 (Pauling); 3.07 (Allrod Rochow)
- Heat of Fusion: 0.3604kJ/mol
- Incompatibilities:
- Ionization Potential
- First: 14.534
- Second: 29.601
- Third: 47.448
- Valence Electron Potential (-eV): 550
Physical Properties of Nitrogen
Atomic Mass For Nitrogen
- Atomic Mass Average: 14.00674
- Boiling Point: 77.5K -195.65°C -320.17°F
- Coefficient of lineal thermal expansion/K-1: N/A
- Conductivity
- Electrical:
Thermal: 0.0002598 W/cmK
- Electrical:
- Density: 1.2506g/L @ 273K & 1atm
- Description:
- Colorless, odorless, tasteless gas
- Enthalpy of Atomization: 472.8 kJ/mole @ 25°C
- Enthalpy of Fusion: 0.36 kJ/mole
- Enthalpy of Vaporization: 2.79 kJ/mole
- Flammablity Class:
- Freezing Point:see melting point
- Heat of Vaporization: 2.7928kJ/mol
- Melting Point: 63.29K -209.86°C -345.75°F
- Molar Volume: 17.3 cm3/mole
- Optical Refractive Index: 1.000298 (gas) 1.197 (liquid)
- Physical State (at 20°C & 1atm): Gas
- Specific Heat: 1.04J/gK
Atomic Mass And Atomic Number For Nitrogen
Regulatory / Health

The Atomic Number For Nitrogen
- CAS Number
- 7727-37-9
- OSHAPermissible Exposure Limit (PEL)
- No limits set by OSHA
- OSHA PEL Vacated 1989
- No limits set by OSHA
- NIOSHRecommended Exposure Limit (REL)
- No limits set by NIOSH
- Levels In Humans:
Note: this data represents naturally occuring levels of elements in the typical human, it DOES NOT represent recommended daily allowances.- Blood/mg dm-3: 34300
- Bone/p.p.m: 43000
- Liver/p.p.m: 72000
- Muscle/p.p.m: 72000
- Daily Dietary Intake: n/a
- Total Mass In Avg. 70kg human: 1.8 kg
- Discovery Year: 1772
- Name Origin:
- Greek: zôê (vie).
- Abundance of Nitrogen:
- Earth's Crust/p.p.m.: 25
- Seawater/p.p.m.:
- Atlantic Suface: 0.00008
- Atlantic Deep: 0.27
- Pacific Surface: 0.00008
- Pacific Deep: 0.54
- Atmosphere/p.p.m.: 780900
- Sun (Relative to H=1E12): 8.71E+07
- Sources of Nitrogen:
- Nitrogen can be made by liquification and then fractional distillation of the air. It is very easily done commercially. It can also be made by heating NaN3 to 300 degrees C. Annual world wide production is around 44,000,000 tons.
- Uses of Nitrogen:
- Nitrogen has many industrial uses in the gaseous forms, but probably the most interesting is liquid nitrogen, which is extremely cold. Items that must be frozen to extremely low temperatures for preservation are frequently stored in liquid nitrogen. Fertility clinics store sperm, eggs and embryos used to help infertile couples become pregnant in ampoules in liquid nitrogen.Since nitrogen gas is very stable, at standard temperature and pressure, it is used as the air in inert welding atmospheres. Documents, foods and chemicals are sometimes stored in nitrogen to keep them from oxidizing or reacting with air or water.
- Additional Notes:
Nitrogen in the elemental form was considered to be inert and was even named ozote which refers to the fact that it is not reactive. Of course nitrogen does form compounds, but the gaseous form consists of diamers (2 nitrogens bonded together). The diamer is very stable.
Nitrogen is a major element in organic compounds, especially proteins. Some nitrogen compounds are highly reactive. Trinitrotoluene is TNT or dynamite. Ammonium Nitrate is a fertilizer, but was used as the major explosive ingredient in the Oklahoma City bombing. Anfo, or Ammonium Nitrate and fuel oil mixture is the primary explosive used in the mining industry because it is inexpensive, easy to manufacture and can be easily manufactured near the mine site thus reducing the risks and expenses related to the transportation of explosives. Nitrates, Nitrites and Azides (all nitrogen compounds are either oxidizers or reactives and will react violently under the right conditions.
Nitrogen Menu
- Nitrogen Page One
- Nitrogen Page Two
- Nitrogen Page Three
References
A list of reference sources used to compile the data provided on our periodic table of elements can be found on the main periodic table page.
Symbol For Nitrogen
Related Resources
- Anatomy of the Atom
Answers many questions regarding the structure of atoms. - Molarity, Molality and Normality
Introduces stoichiometry and explains the differences between molarity, molality and normality. - Molar Mass Calculations and Javascript Calculator
Molar mass calculations are explained and there is a JavaScript calculator to aid calculations. - Chemical Database
This database focuses on the most common chemical compounds used in the home and industry.
Citing this page
If you need to cite this page, you can copy this text:
Kenneth Barbalace. Periodic Table of Elements - Nitrogen - N. EnvironmentalChemistry.com. 1995 - 2021. Accessed on-line: 4/25/2021
https://EnvironmentalChemistry.com/yogi/periodic/N.html/
.
Linking to this page

If you would like to link to this page from your website, blog, etc., copy and paste this link code (in red) and modify it to suit your needs:
<a href='https://EnvironmentalChemistry.com/yogi/periodic/N.html/'>echo Periodic Table of Elements: Nitrogen - N (EnvironmentalChemistry.com)</a>- Comprehensive information for the element Nitrogen - N is provided by this page including scores of properties, element names in many languages, most known nuclides and technical terms are linked to their definitions.
.
NOTICE: While linking to articles is encouraged, OUR ARTICLES MAY NOT BE COPIED TO OR REPUBLISHED ON ANOTHER WEBSITE UNDER ANY CIRCUMSTANCES.
PLEASE, if you like an article we published simply link to it on our website do not republish it.
